Pharma Wisdom Jobs
23:17:00
0
For View full Power Point Presentation of This Topic
Historical
development of Sulfonamides:
Building on Ehrlich’s early work, Gerhard
Domagk, a medical doctor employed by a German dye manufacturer made a
breakthrough discovery by finding that a dye known as prontosil, dosed orally,
was effective in curing life threatening streptococci infections in humans. He
made the discovery in a desperate, but successful attempt to save his daughter
who was dying of a streptococci infection.
German bacteriologist and pathologist who was awarded the 1939
Nobel Prize for Physiology or Medicine for his discovery (announced in 1932) of
the antibacterial effects of Prontosil, the first of the sulfonamide drugs.
Chemistry of Sulfonamides:
- Recognized
since 1932.
- In clinical usage since 1935.
First compounds found to be effective
antibacterial agents in safe dose ranges.
- Chemically, it is a molecule
containing the sulfonamido (sulfanilamide, SO2NH2) functional group attached to aniline.
- Structurally related to p-amino benzoic
acid (PABA).
- Chemical Modification of the sulphonamide
structure has given rise to several important groups of drugs.
- This
group is also present in other non-antibacterial compounds like
Oral Hypoglycemic – Sulphonyl ureas
Diuretics – Thiazides (Furosemide)
Anti Mycobacterial - Sulphones
Glucoma -Acetazolamide
- All sulfa
are white crystalline powder except Sulfaquinoxaline (yellow).
- Sulfa generally are weak organic acid,
insoluble in water but much more soluble in alkaline aqueous sol. than in
neutral or acidic.
- The spectrum of all sulfonamides is generally
the same.
- Sulfonamide inhibit
both Gram-positive & Gram negative bacteria, Nocardia, Actinomyces spp,
& some Protozoa as Coccidia & Toxoplasma spp. & Streptococcus, Staphylococcus,
Salmonella, Pasteurella, & E. coli .
- Sulfa used to treat or prevent acute systemic
or local infections.
Mechanism of Sulfonamides :
- Sulfonamide
molecular structure is similar to p-Amino benzoic acid (PABA) which is
needed in bacteria organisms as a substrate of the enzyme dihydro pteroate
synthetase for the synthesis of Tetra Hydro Folic acid (THFA).
- Folic acid - synthesized
from PABA, pteridine and glutamate.
- All sulfonamides are analogs of PABA.
- All sulfa drugs
are bacteriostatic.
SAR of Sulfonamides:
Structure of Sulphonamides could be divided in to four parts:
1.)
Para amino group
2.)
Aromatic Ring
3.)
Sulphonamide group
4.)
N1-Substitution
1. Para Amino Group:
- The para
amino group is essential for the activity and must be unsubstituted.
- It should be
always substituted on para position of aromatic ring other wise anti bacterial
activity is lost.
2. Aromatic Ring:
- It is the
minimal structural requirement for the antibacterial activity.
- It should be always para substituted.
- Replacement of Aromatic ring by other
ring systems or the introduction of additional substituents on it decreases or
abolish activity.
3. Sulphonamide group:
- Sulphonamide group along with aromatic ring is
essential for the antibacterial activity.
- Sulphur atom should be directly linked to
aromatic ring.
- The amino & Sulphonyl groups on
the benzene ring are essential & should be in 1,4-position.
- Exchange of
the -SO2NH group by –CO-NH reduce the activity.
4. N1-Substitution:
- Sulphonamide nitrogen should be primary or
secondary.
- R could be substituted
with hydrogen, aromatic ring or heterocyclic ring.
- Substitution
of Aromatic Heterocyclic nuclei at N1 - yields highly potent
compounds.
- N1 –Di substitution in general
leads to inactive.
Classification of Sulphonamides:
Sulfonamide Structures:
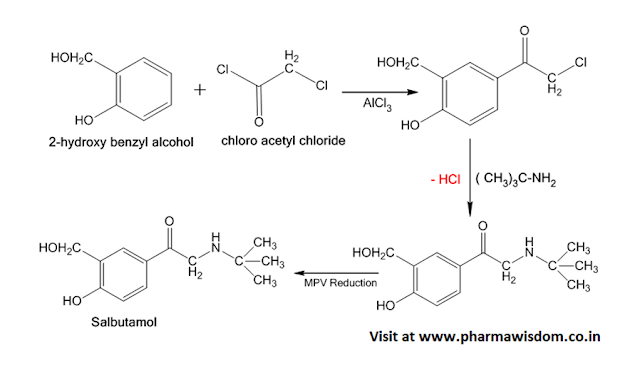
Synthesis of Sulfacetamide:
Synthesis of
Adverse Effects:
- Produce
mild-to-moderate nausea, vomiting, headache and mental depression.
- Produce hypersensitivity reactions (rashes,
fever, eosinophilia).
- Rarely cause Stevens-Johnson Syndrome,
erythema multiforme associated with lesions of skin and mucous membranes.
- Produce Kernicterus (bilirubun-induced
brain dysfunction) in neonates because of the displacements of bilirubin form
serum albumin binding site.
- Sever
adverse effects includes hepatitis, bone marrow depression and
crysalluria
Folate Reductase Inhibitors:
TRIMETHOPRIM:
- Trimethoprim is selective inhibitor of bacterial DHFR
- Individually they both are bacteriostatic but the combination is bactericidal
CO-TRIMOXAZOLE:
Fixed dose combination
SULPHAMETHAXAZOLE : TRIMETHOPRIM
(5 : 1)
- SEQUENTIAL
BLOCK.
- Broad
spectrum bactericidal combination.
- Delays the
development of bacterial resistance
Synthesis of Trimethoprim:
Sulfones:
A sulfone is
a chemical compound containing a sulfonyl functional group attached to two
carbon atoms. The central hexavalent sulfur atom is double-bonded to each of
two oxygen atoms and has a single bond to each of two carbon atoms, usually in
two separate hydrocarbon substituents.
Dapsone
(diamino diphenyl sulfone, DDS), It was discovered by German chemists
Fromm and Wittmann in 1908, Was not
utilized as a treatment until decades later.
Dapsone is used to treat dermatitis herpetiformis (a skin
condition) and leprosy.
It is also used with other drugs to treat Hansen's
disease.
Dapsone
is commonly used in combination with rifampicin and clofazimine for
the treatment of leprosy
Synthesis of Dapsone:
Note: All information copyright @ www.pharmawisdom.co.in
Prepared By:
S.Seetaramswamy, M.Pharm
Assoc. Prof.
Dept. Pharmaceutical Chemistry
Prepared By:
S.Seetaramswamy, M.Pharm
Assoc. Prof.
Dept. Pharmaceutical Chemistry
For More Pharma Study Materials - Click Here
Join for Regular Job Updates in Whats App & Telegram...
Thank You for Visiting