Pharma Wisdom Jobs
00:22:00
14
Full Topic PPT - CLICK HERE
Anthelmintics
are drugs that either kill (vermicide) or expel (vermifuge) infesting
helminths.
The helminths comprise two major Groups:
1.
Nemathelminths (Nematodes, roundworms)
2.
Platyhelminths (Flatworms)
The
latter group is subdivided into the two groups
a)
Trematode (Flukes)
b)
Cestodes (Tapeworms)
Almost
350 species of helminths have been found in humans, and most colonise the
gastrointestinal tract (G.I.T).
Humans
are primary host for helminth infections, in the sense that they harbour the
sexually mature form that reproduces. Eggs or larvae then pass out of the body
and infect the secondary (intermediate) host.
Helminthiasis
is more common in developing countries with poorer personal and environmental
hygiene.
They
harm the host by depriving him of food, causing blood loss, injury to organs,
intestinal or lymphatic obstruction and by secreting toxins. Helminthiasis is
rarely fatal, but is a major cause of ill health.
Classification of Anthelmintic Agents:
1) Based on Chemical Structure
2) Based on Pharmacology (Chemotherapy)
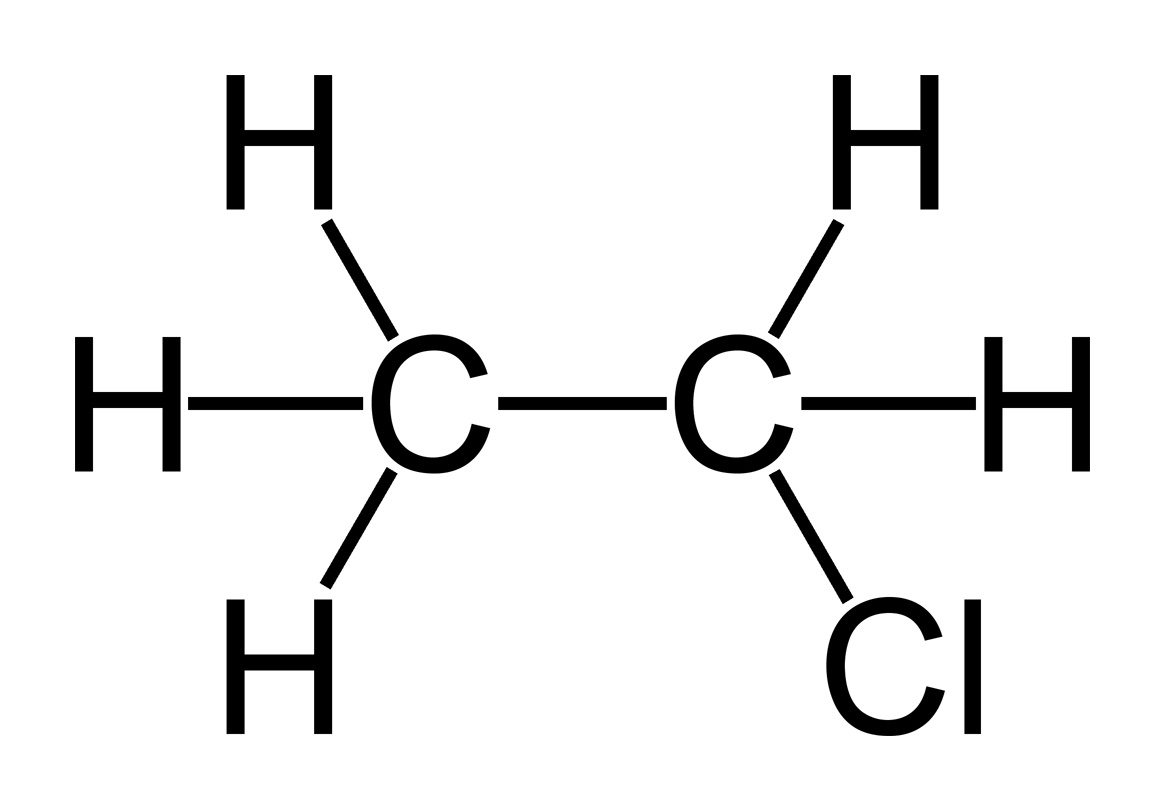
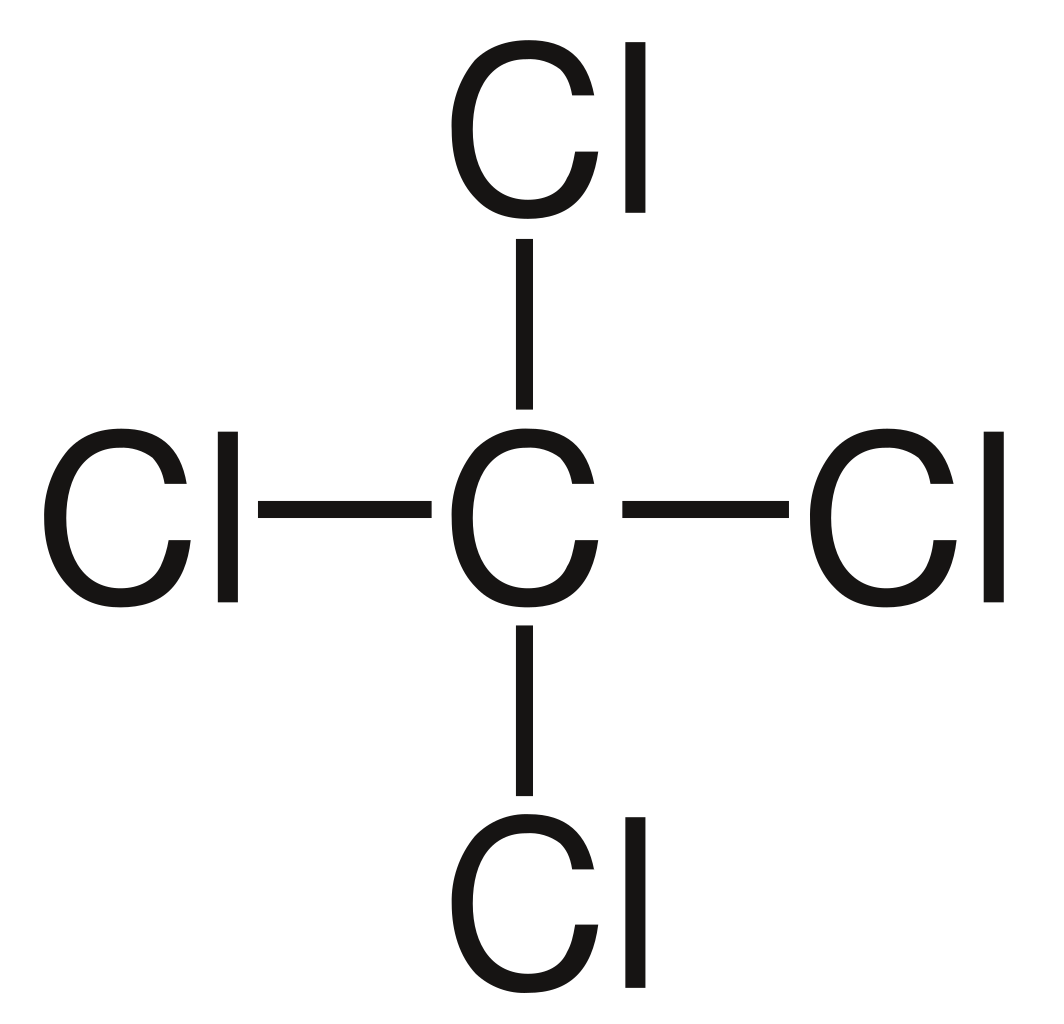
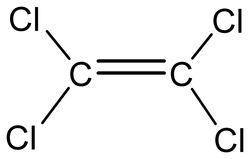
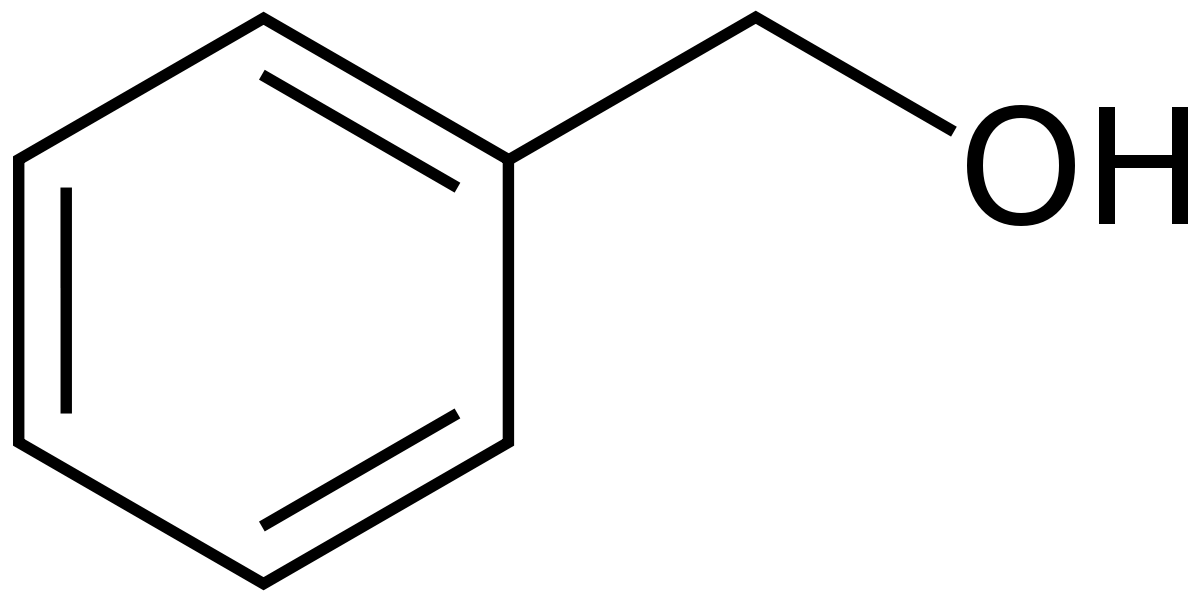
Some Important structures of Anthelmintic Agents
The
first of this class, Thiabendazole, was discovered in 1961 and
subsequently a number of further Benzimidazoles were introduced as broad
spectrum Anthelmintics. Thiabendazole effective against threadworm, cutaneous
larva migrans, and early stage of trichinosis. Thiabendazole has
anti-inflammatory, analgesic and antipyretic actions.
Mebendazole is a synthetic benzimidazole that has a wide spectrum of
Anthelmintic activity and a low incidence of adverse effects. It is a drug of
choice in the treatment of infections by whipworm eggs, pinworm, hookworms, and
roundworm.
Albendazole, a broad-spectrum oral Anthelmintic agent. It is the drug of
choice for treatment of hydatid disease and cysticercosis. It is also used in
the treatment of pinworm and hookworm, round worm, whip worm, and thread worm
infections.
Synthesis of Diethylcarbamazine citrate:
Synthesis of Mebendazole:
MOA of Anthelmintic Agents:
Side Effects of Anthelmintic Agents:
Note: All information copyright @ www.pharmawisdom.co.in
Prepared By:
S.Seetaramswamy
Assoc. Prof.
Dept. Pharmaceutical Chemistry
Read More Pharma Topics - CLICK HERE
Join for Regular Job Updates in Whats App & Telegram...
Thank You for Visiting
More Updates Visit daily @ www.pharmawisdom.co.in