Unit-III: Alkyl Halides
Read full PPT of this Topic - CLICK HERE
Alkyl halides are also known as haloalkanes.
Alkyl halides are compounds in which one or more
hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine,
chlorine, bromine or iodine).
Alkyl halides are represented by R-X (Where
R-alkyl group, X- F, Cl, Br or I
Types of Organic Halides:
Alkyl Halides: Have a halogen atom
(X) bonded to a C-C Bond.
Vinyl Halides: Have a halogen atom
(X) bonded to a C=C Bond.
Aryl Halides: Have a halogen atom (X) bonded to a Ar-C
Bond.
Classification of Alkyl Halides:
The number of alkyl groups attached to the carbon to which the
halogen is bonded determines whether an alkyl halide is Primary, Secondary or
Tertiary.
Primary (1°) Alkyl Halides: have
one R group attached to the carbon linked to the halogen.
Secondary (2°) Alkyl Halides: have
two R groups attached to the carbon linked to the halogen.
Tertiary (3°) Alkyl Halides: have
three R groups attached to the carbon linked to the halogen.
Nucleophilic Substitution Reactions (SN1 and SN2)
A nucleophile, a species with an unshared electron
pair (lone-pair electrons), reacts with an alkyl halide (substrate) by
replacing the halogen substituent (leaving group) this is known as Nucleophilic
Substitution Reaction.
In Nucleophilic substitution reactions, the C–X bond of the
substrate undergoes heterolysis, and the lone-pair electrons of the
nucleophile is used to form a new bond to the carbon atom.
A nucleophile (“nucleus loving”, or
“positive-charge loving”) is a reactant that provides a pair of electrons to
form a new covalent bond.
There are Two types of Nucleophilic substitution reactions called
1) SN1 : Unimolecular Nucleophilic Substitution
2) SN2 : Bimolecular Nucleophilic Substitution
SN1 Reaction:
Characteristics of SN1 Reaction
- Two-step
reaction process: 1. carbon-halogen bond breaks,
resulting in a positively charged carbon (carbocation) and 2. nucleophile
attacks the carbocation, forming a new bond
- Unimolecular
and follows first-order kinetics
- Rate of
the reaction depends on the concentration of the substrate (alkyl halide)
- Has a
racemization stereochemistry, i.e., both retention and inversion products
are formed
- Polar
protic solvent is used to enhance the reactivity
- Mostly occurs in tertiary and secondary alkyl halide, with the former having a higher reaction rate than the latter
SN2 Reaction:
Characteristics of SN2 Reaction
- One-step
reaction process, the reaction is concerted: carbon-halogen bond breaking
and carbon-nucleophile bond formation takes place at the same time.
- Bimolecular
and follows second-order kinetics.
- Rate of
the reaction depends on the concentrations of the substrate (alkyl halide)
and the nucleophile.
- Has an
inversion (Walden
inversion) stereochemistry at the reaction center.
- Polar
aprotic solvent is used to enhance the reactivity
- Mostly
occurs in primary and secondary alkyl halides
SN1 versus SN2 reactions:
Factors affecting SN1 and SN2 reactions:
The rate of an SN1 & SN2 reaction depends upon 4 factors:
1. The nature of the substrate (the alkyl halide)
2. The power of the nucleophile
3. The ability of the leaving group to leave
4. The nature of the solvent
Nature of the substrate (the alkyl halide):
In
SN1 & SN2 reaction the substrate plays the most important part in
determining the rate of the reaction. Highly substituted alkyl halides
(substrates) form a more stable Carbonium ion. 3° alkyl halides undergo SN1 reactions with ease. 1° alkyl halides undergo SN2 reactions with
ease.
Nature of the Nucleophile :
• A nucleophile with a negative charge is
always more powerful than its conjugate acid.
Thus
OH¯ is more powerful than H2O & NH2¯ is more
powerful than NH3 & CH3O¯ is more powerful than CH3OH,
etc.
• The weak nucleophile favors an SN1
mechanism
• The strong nucleophile favors an SN2
mechanism
Nature of the leaving group:
•
The nature of the leaving group has the same effect on both SN1 and SN2
reactions.
•
The better the leaving group, the faster a C+ can form and hence the faster
will be the SN1 reaction.
•
The leaving group usually has a negative charge
Example: Iodine (-I)
is a good leaving group because iodide (I-) is non basic.
The
hydroxyl group (-OH) is a poor leaving group because hydroxide (OH-) is a
strong base.
The nature solvent :
There
are 3 classes of organic solvents:
Protic
solvents: which contain –OH or –NH2 groups.
Polar
aprotic solvents: which contain strong dipoles but no –OH or –NH2 groups.
Non
polar solvents: e.g., hydrocarbons.
Polar protic solvents like H2O and ROH favor SN1
reactions because the ionic intermediates (both cations and anions) are
stabilized by solvation.
Polar aprotic solvents favor SN2 reactions
because nucleophiles are not well solvated, and therefore, are more
nucleophilic.
Ethylchloride:
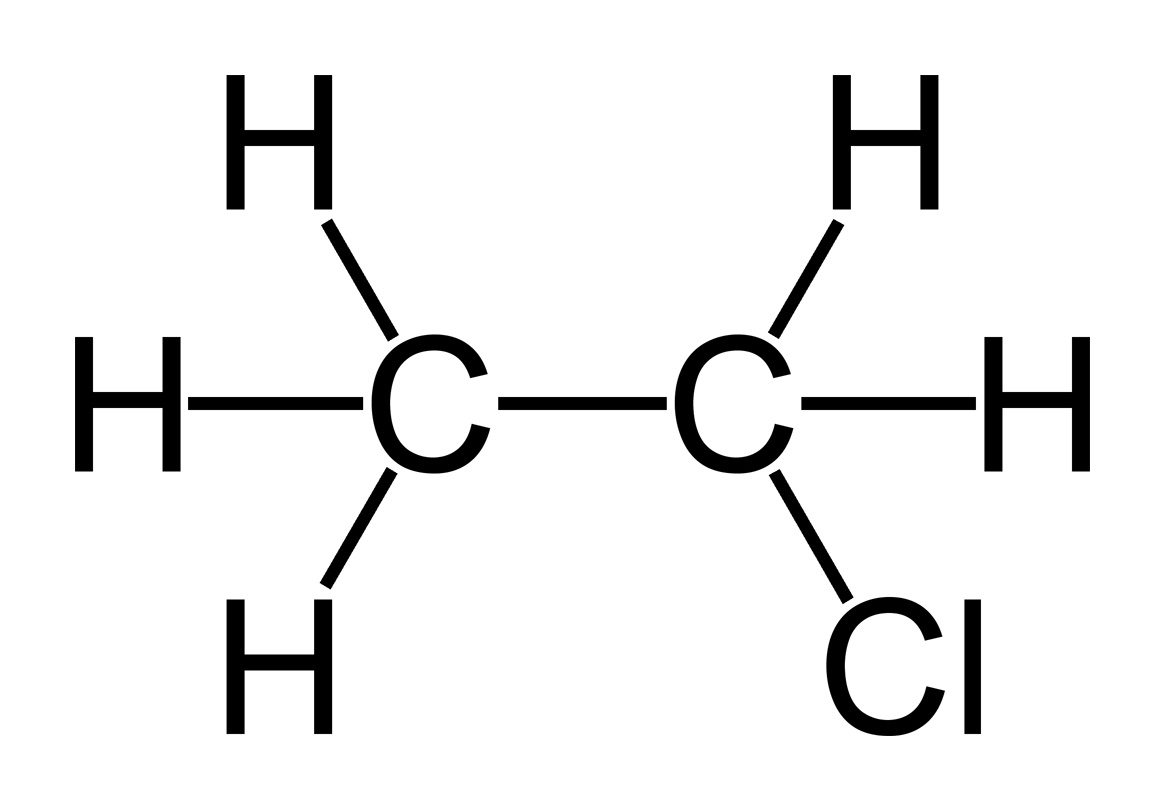
Chloroethane or
monochloroethane, commonly known by its old name ethylchloride, is a chemical
compound with chemical formula C2H5Cl, once widely used
in producing tetraethyllead, a gasoline additive.
Chloroethane is
produced by hydro chlorination of ethene:
C2H4 + HCl → C2H5Cl
Uses:
- The major use of chloroethane was to
produce tetraethyllead (TEL), an anti-knock additive for gasoline.
- It
is used in the production of cellulose, dyes, medicinal drugs, and other
commercial products, and as a solvent and refrigerant.
Chloroform:

Chloroform or
trichloromethane is an organic compound with formula CHCl3. It is a
colorless, sweet-smelling, dense liquid.
Uses:
- It is used as a
solvent for lacquers, floor polishes, resins, adhesives, alkaloids, fats, oils
and rubber.
- Chloroform is used in making Fluorocarbon- 22,
a refrigerant.
- Chloroform is also
used to extract and purify penicillin.
- Chloroform used for
extraction and purification of Alkaloids.
Trichloroethylene:

The chemical
compound trichloroethylene is a halocarbon commonly used as an industrial
solvent. It is a clear non-flammable liquid with a sweet smell.
When1, 2-dichloroethane
heated with chlorine at 400°C is produced to trichloroethylene.
ClCH2CH2Cl + 2Cl2
→ ClCH=CCl2 + 3HCl
Uses:
- The main use of
trichloroethylene is in the vapor degreasing of metal parts.
- Trichloroethylene
is also used as an extraction solvent for greases, oils, fats, waxes, and tars,
a chemical intermediate in the production of other chemicals, and as a
refrigerant.
Tetrachloromethane:
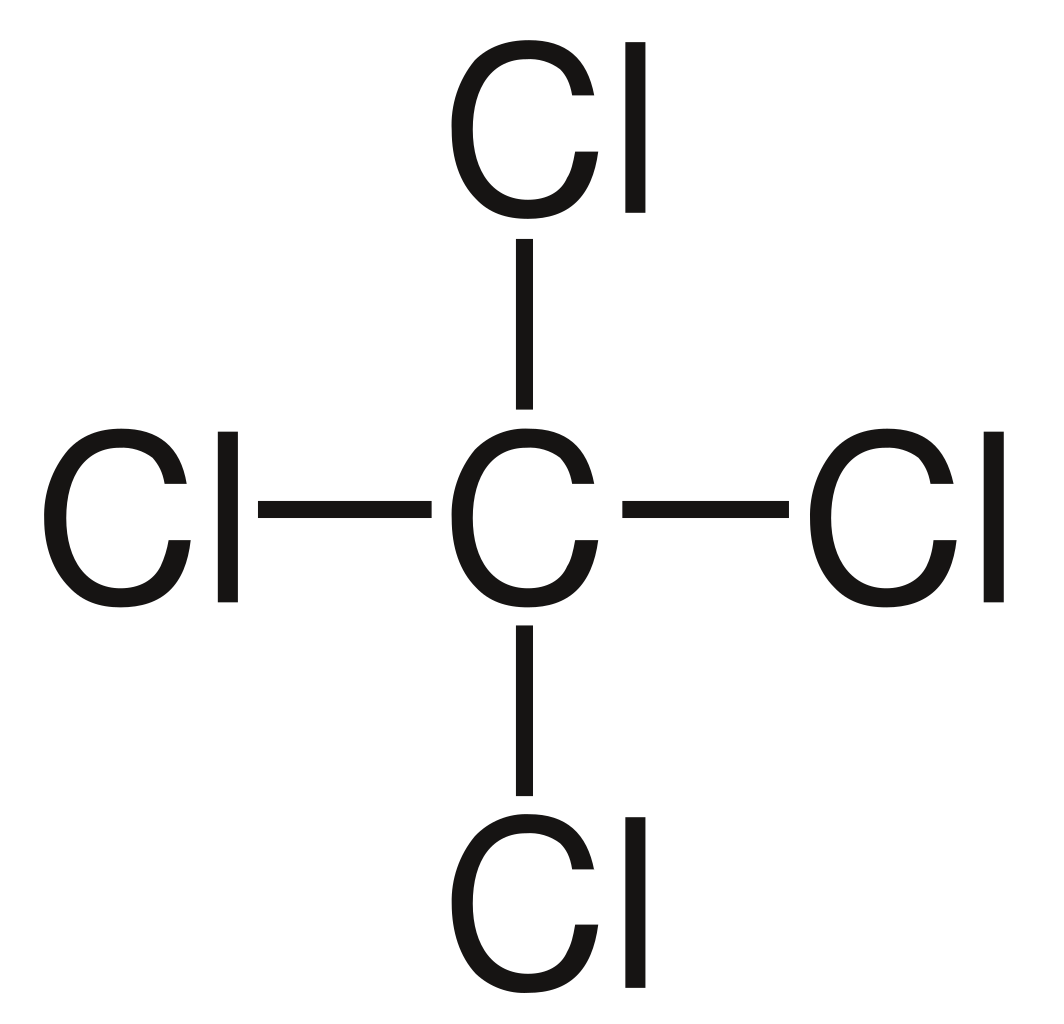
- Tetrachloromethane
also known by many other names Carbon tetrachloride (CCl4).It is a colourless liquid with a "sweet" smell that
can be detected at low levels.
It is mainly
produced from methane:
CH4 + 4 Cl2 → CCl4 + 4 HCl
Uses:
- Carbon tetrachloride
was used to produce the chlorofluorocarbon refrigerants R-11 (trichlorofluoromethane)
and R-12 (dichlorodifluoromethane).
- Carbon tetrachloride
has also been used in the detection of neutrinos.
- It is a useful
solvent for halogenations.
- Carbon tetrachloride
was widely used as a dry cleaning
solvent, and metal degreasing solvent.
Tetrachloroethylene:
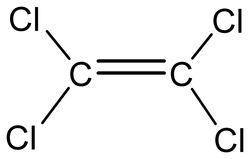
Tetrachloroethylene is a volatile chlorinated organic hydrocarbon
that is widely used as a solvent with the chemical formula C2Cl4.
It is non-flammable liquid at room temperature, evaporates easily into the air
and has a sharp sweet odour.
It is prepared by
thermal decomposition of hexachloroethane.
C2Cl6 → C2Cl4 + Cl2
Uses:
- Tetrachloroethylene
is an excellent solvent for organic materials.
- It is widely used
in dry cleaning.
- It is also used to
degrease metal parts in the automotive and other metalworking
industries, usually as a mixture with other chlorocarbons.
Dichloromethane:

Dichloromethane (DCM
or methylene chloride) is an organic compound with the formula CH2Cl2.
This colorless, volatile liquid with a moderately sweet aroma.
DCM is produced by
treating chloromethane with chlorine gas at 400–500 °C.
CH3Cl + Cl2 → CH2Cl2 + HCl
Uses:
- DCM is a useful
solvent for many chemical processes.
- It is widely used
as a paint stripper and a degreaser.
- In the food
industry, it has been used to decaffeinate coffee and tea as well as to prepare
extracts of hops and other flavorings.
Iodoform:
It is also known as
tri-iodomethane, carbon triiodide, and methyl triiodide.
Iodoform is the
organoiodine compound with the formula CHI3. A pale yellow,
crystalline, volatile substance, it has a penetrating and distinctive odor and,
analogous to chloroform, sweetish taste.
CH3CH2OH + 6NaOH+4I2
→ CHI3 +HCOONa +5H2O
+5NaI
CH3COCH3 + 4NaOH+3I2
→ CHI3 +CH3COONa +3H2O
+3NaI
Uses:
It is occasionally
used as a disinfectant.
It was used in
medicine as a healing and antiseptic dressing for wounds and sores.
Alcohols:
Read full PPT of this Topic - CLICK HERE
Alcohols
is any organic compound in which the hydroxyl functional group (-OH) is bound
to a saturated carbon atom.
The
organic compound which has -OH functional group are called alcohols.
The
general formula for alcohol is CnH2n+1OH / R-OH.
Classification of Alcohol:
On
the basis of -OH group attached to the carbon atom, alcohols are divided into
three categories:
Primary alcohol: When the carbon atom attached to the hydroxyl
group is bonded to only one carbon atom such type of alcohol is known as primary
alcohol.
Secondary alcohol: When it is bonded to two carbon atoms such
type of alcohol is known as secondary alcohol.
Tertiary alcohol: When it is bonded to three carbon atoms such
type of alcohol is known as tertiary alcohol.
Quality Test for Alcohols:
The
following tests may be used to detect the presence of an –OH group in
organic compounds.
For
these tests, take the liquid compound or a solution of solid compound in an
inert solvent such as dry ether or benzene.
A)
Esterification Test:
B)
Sodium metal test
C)
Ceric ammonium nitrate test
D)
Acetyl chloride test
E)
Iodoform test
A)
Esterification Test:
Carboxylic
acid reacts with alcohols forming a fruit smelling ester. The reaction between
an alcohol and a carboxylic acid is called Esterification.
This
reaction is a slow reaction catalysed by concentrated sulfuric acid.
The
chemical reaction is given below.
R-OH + R-COOH → R-COOR + H2O
CH3OH + CH3-COOH → CH3-COOCH3 +
H2O
Note: A sweet smell indicates the presence of alcoholic group.
B) Sodium
Metal Test:
It
is based on the appearance of brisk effervescence due to the
liberation of hydrogen gas when alcohol reacts with active metals like sodium.
The
chemical reaction is given below.
2R-OH + 2Na → 2R-O-Na + H2↑
2CH3-OH + 2Na → 2CH3-O-Na + H2↑
The
alcohol to be tested should be dry because water also reacts with sodium.
Sodium should be handled carefully, unreacted sodium should be destroyed by
adding excess alcohol. This test is favorable if phenyl or carboxyl
groups are absent.
Note: Evolution of hydrogen gas cause a brisk effervescence
indicates an alcoholic group.
C) Phosphorus Pentachloride Test:
Alcohol reacts with PCl5 results in the
mixture becomes warm with evolution of HCl Gas, the given compound contains an
–OH group.
The chemical equation is given below.
R-OH + PCl5 → R-Cl + POCl3 + HCl
Note: Evolution of HCl gas indicates an
alcoholic group.
D) Ceric Ammonium Nitrate Test:
Alcohol
or reaction with Ceric ammonium nitrate forms a pink or red colour precipitate
due to the formation of a complex compound and ammonium nitrate.
The
chemical reaction is given below.
(NH4)2 [Ce(NO3)6]
+ 3ROH → [Ce(NO3)4(ROH)3] + 2NH4NO3
(NH4)2 [Ce(NO3)6]
+ 3CH3OH → [Ce(NO3)4(CH3OH)3]
+ 2NH4NO3
Note: The appearance of red colour precipitate shows the presence
of alcoholic group.
E) Acetyl Chloride Test:
Alcohol
reacts with acetyl chloride results in the formation of ester and hydrogen
chloride. The resulting hydrogen chloride on contact with ammonium hydroxide
forms a white fumes of ammonium chloride and water.
The
chemical equation is given below.
R-OH + CH3-CO-Cl → CH3-COOR + HCl
HCl + NH4OH → NH4Cl + H2O
Note: The formation of white fumes indicates the presence of
alcohol.
Distinction between Primary, Secondary
and Tertiary Alcohols:
1) Lucas Test: (Lucas reagent - ZnCl2 + Conc.
HCl)
The mixture of zinc
chloride and concentrated hydrochloric acid is called Lucas reagent.
It reacts with primary,
secondary and tertiary alcohols at different rates. This reagent forms a
cloudiness on reacting with alcohols.
Tertiary alcohols reacts immediately and give cloudiness,
secondary alcohols reacts slowly and gives cloudiness after 5 to 10 minutes and
there is no reaction with primary alcohols, this because primary alcohols do
not react with Lucas Reagent at room temp. High temperatures are needed.
2) Oxidation Test (Dichromate Test)
In the oxidation test,
the alcohols are treated with sodium dichromate (Na2Cr2O7)
in sulphuric Acid (orange solution). The rate of oxidation varies between
primary, secondary and tertiary alcohol. On the basis of their oxidation rates,
alcohols can be distinguished as:
Primary alcohol gets easily oxidized to an aldehyde and can further be oxidized to
carboxylic acids too. There will be a change in colour of the solution from
orange to green.
Secondary alcohol gets easily oxidized to ketone but further oxidation is not
possible. There will be a change in colour of the solution from orange to
green.
Tertiary alcohol doesn’t get oxidized in the presence of sodium
dichromate. Solution will remain orange.
3) Victor Meyer test:
Victor Mayer method is one of the most important
methods of identification of alcohols.
In this methods primary, secondary and tertiary
alcohols are subjected to a series of chemical analysis and the colour of
resulting solution is observed.
The different steps involved in Victor Meyer
methods are as below 1. The alcohol is treated with iodine in presence of red
phosphorous to obtain iodol alkane.
2. Iodoalkane so formed is allowed to react with
alcoholic silver nitrate in order to obtained nitroalkane.
3. The nitroalkane is treated with nitrous acid
(the mixture of NaNO2 +HCl) and the resulting solution is made
alkaline.
4. The colour of resulting solution is observed in
which following observations are observed.
a. A primary alcohol gives blood red colour.
b. A secondary alcohol gives the blue colour.
c. A tertiary alcohol does not produce any colour.
Structure and Uses of Alcohols:
Ethyl alcohol:

It is also known as alcohol, Ethanol and drinking
alcohol, is a chemical compound, a simple alcohol with the chemical formula C2H5OH
or CH3−CH2−OH.
Ethanol in
alcoholic beverages and fuel is produced by fermentation. Certain species of
yeast metabolize sugar, producing ethanol and carbon dioxide.
C6H12O6 → 2 CH3CH2OH
+ 2 CO2
Uses:
- Ethanol is used in
medical wipes and most common antibacterial hand sanitizer gels as an antiseptic. Ethanol kills
organisms by denaturing their proteins and is effective against most bacteria, fungi,
and many viruses.
- Ethanol may be
administered as an antidote to methanol
and ethylene glycol poisoning.
Chlorobutanol:

Chlorobutanol
(trichloro-2-methyl-2-propanol), is commonly used as a chemical preservative in
a wide range of cosmetic and pharmaceutical products.
Chlorobutanol is
formed by the simple Nucleophilic addition of chloroform and acetone in
presence of KOH / NaOH.
Uses:
- Topically along
with clove oil as dental analgesic.
- Chlorobutanol has
been employed as sedative and hypnotic.
- Chlorobutanol used
as pharmaceutical aid (antimicrobial) antiseptic and local anesthetic.
Cetostearyl alcohol:
It is also known as
cetearyl alcohol or cetylstearyl alcohol is a mixture of fatty alcohols,
consisting predominantly of CETYL and STEARYL ALCOHOLS and is classified as a
fatty alcohol.
Uses:
- Used in the
cosmetic industry as an opacifier in shampoos, or as an emollient, emulsifier
or thickening agent in the manufacture of skin creams and lotions.
- It is also
employed as a lubricant for nuts and bolts, and is the active ingredient in
some "liquid pool covers" (forming a surface layer to reduce
evaporation and retain heat).
Benzyl alcohol:
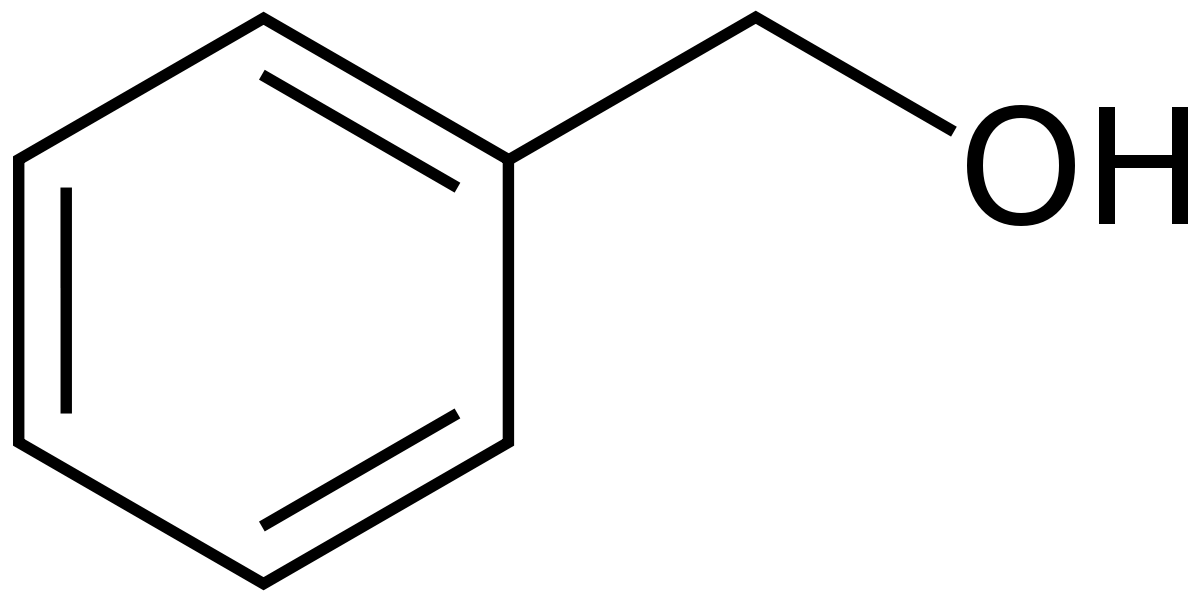
Benzyl alcohol is an aromatic alcohol with the formula C6H5CH2OH.
Benzyl alcohol is
a colorless liquid with a mild pleasant aromatic odor.
Benzyl alcohol is
prepared by the hydrolysis of benzyl chloride using KOH / NaOH:
Uses:
Benzyl alcohol is
used as a general solvent for inks, waxes, shellacs, paints, lacquers, and
epoxy resin coatings.
It is also used in
e-liquid for e-cigarettes to enhance the flavors used.
Glycerol:

It is also called glycerine or glycerin.
It is a colorless, odorless, viscous liquid that is sweet-tasting and
non-toxic. The glycerol backbone is found in those lipids
known as glycerides.
Uses:
- Glycerol having antimicrobial and antiviral properties it is widely
used in FDA approved wound and burn treatments.
- It can also be used as an effective marker to measure liver disease.
- It is also widely used as a sweetener in the food industry
Propylene glycol:

Propylene glycol (propane-1,2-diol)
is an organic compound with the chemical formula CH3CH(OH)CH2OH.
It is a viscous, colorless liquid, which is nearly odorless but
possesses a faintly sweet taste.
Uses:
- Propylene glycol is a synthetic liquid substance
that absorbs water.
- Propylene glycol is also used to make polyester
compounds, and as a base for deicing solutions.
- Propylene glycol is used by the chemical, food,
and pharmaceutical industries as antifreeze when leakage might lead to contact
with food.
Prepared By:
Note: All information copyright @ www.pharmawisdom.co.in
Prepared By:
S.Seetaramswamy
Assoc. Prof.
Dept. Pharmaceutical Chemistry
Join for Regular Job Updates in Whats App & Telegram...
Thank You for Visiting
More Updates Visit daily @ www.pharmawisdom.co.in

















I am so thankful for your content, I have a list of amazon products like alcohol prep pads which will improve your lifestyle.
ReplyDeleteMantram Nursing Academy’s online coaching for BSc Nursing entrance exams offers students live sessions, recorded lectures, and regular assessments. This ensures a comprehensive and flexible study plan for entrance exam success.
ReplyDeleteOnline Coaching for BSc Nursing Entrance Exam In Chandigarh
good information nice post..
ReplyDeletePrednisone
Semaglutide
Estradiol
https://pharmawisdom.blogspot.com/2020/04/alkyl-halides-alcohols-unit-3-bpharm.html?sc=1772526456043#c8847740863126808333
ReplyDelete