Wednesday 24 February 2021
NOVICK BIO-SCIENCES - Walk-In Drive for Freshers & Experienced in Formulation R&D on 27th Feb' 2021
Tuesday 23 February 2021
SMS Pharmaceuticals Limited - Walk-In Interviews for Freshers & Experienced in Production (20 Positions) on 25th & 26th Feb' 2021
Walk-In
Interviews @ SMS Pharmaceuticals Limited
Production -
B.Sc / M.Sc (0-4 Years) 20 No’s
(Above all
positions are must having API Pharma experience)
Above all positions walk-in interview conducted 25th &
26th February 2021, Preferable immediately
joining.
Candidate must carry 1) Resume 2) Three Months’ Pay slips 3) Six months
bank statement 4) Pass port size photo.
Male Candidate only.
Interview
Venue:-
SMS Pharmaceuticals Limited
Unit-II: Plot No.24 & 24 B and 36 & 37,
S.V. Co - Operative Industrial Estate, Bachupally,
Medchal-Malkajgiri District, Hyderabad-500 090,
Telangana, INDIA.
Email: admin_unit2@smspharma.com
Immediate Openings for Freshers & Experienced in Quality Control / Production / FR&D - Formulation / Bulk Company @ Hyderabad
Greetings of the KRISHNAVENI PHARMA CONSULTANCY Jobs...!!!
Immediate Openings for
Executive: FR&D
(Formulation Company)
Location: Jeedimetla
Experience: Freshers
Qualifications: M.Pharmacy (2018, 2019, 2020 Batches) Only Males
Ctc : 13,500/-
Vacancies: 8
Executive: Q.C
(Formulation Company)
Location: Uppal Area
Experience: Freshers
Qualifications: M.Pharmacy, B.Pharmacy, M.Sc, B.Sc
(2017, 2018, 2019, 2020 Batches) Only Males
Ctc : 8000/-(Increment Year Wise)
Vacancies: 8
Sr. Executive:
QC (Formulation Company)
Exp: 2yrs
Location: Pragathi Nagar (Aleap Industrial Area)
Ctc : Negotitable
Vacancies: 5
Executive: QC
(Formulation Company)
Location: Cherlapally
Exp: Experinced2 -4 Years
Ctc : Negotiable
Vacancies
Executive:
QC (Bulk Company)
Location: Nacharam
Exp: Experinced1-4 Years
Ctc : Negotiable
Vacancies: 5
Executive:
Production (Bulk)
Location: Medchal
Exp : Experinced;1 &3 Years
Ctc : Negotiable
Vacancies : 4
Interested persons contact on Below Details
Mobile: 8885637856,
9652169734, 9581679067, 04048530335
Mail ID: krishnavenipharmaconsultancy@gmail.com
Maithri Drugs Pvt. Ltd - Walk-In Drive for M.Sc / M.Pharm / B.Sc / B.Pharm Candidates - Quality Control on 24th Feb' 2021
Maithri
Drugs Pvt. Ltd, Bonthapally: QC Department
walk-in drive on 24/02/2021
VACANCIES: Junior Executive's/ Executive's/ Senior Executive's
QUALIFICATION: M.Sc / M.Pharm / B.Sc / B.Pharm
ELIGIBILITY CRITERIA: 3-6 yrs of work experience - Only MEN candidates
JOB REQUIREMENTS:
1. Need to posses sound knowledge in handling instruments like HPLC , GC, IR,
UV, Potentiometer etc.,
TIMINGS: Interested candidates may walk-in from 09:00 AM to
01:00 PM
DOCUMENTS TO BE CARRIED: Candidates are requested to carry updated
resume, photocopies of educational certificates, latest increment letter, last
3 months pay slips, last 6 months bank statement and photocopy of aadhar card.
INTERVIEW VENUE & WORK LOCATION: Maithri Drugs Pvt Ltd,
Bonthapally. Landmark: Bonthapally Kamaan, Beside
Bharat Petroleum Bunk Lane.
GOOGLE MAP: https://goo.gl/maps/vV9L5cG9vadJVZS87
Contact: 040 30438366, 08455 694101
Monday 22 February 2021
60 Openings | Walk-In Drive for Freshers & Experienced in Analytical R&D on 25th Feb' 2021 @ MSN Laboratories Pvt. Ltd
Walk- In Drive for Analytical Research & Development (AR&D
- API) - Method Development & Method Validation, Fresher's & Experience Department
.in API Division- R&D Center.
Openings: 60
Experience
AR&D Method Development & Method Validation / NMR / GCMS / LCMS / HPLC /
GC / Mass Spectrometry / Ion Chromatography /Preparative- HPLC / NMR/Peptide
AR&D Department - API. .in API
Division-R&D Center.
Experience:
0 to 8 Years API - AR&D Method Development & Method Validation
Department: Analytical R&D (AR&D - API)
(Analytical Research & Development) Method Development & Method
Validation Analytical Research & Development -API
Qualification:
M.Sc Analytical Chemistry Fresher's - 2018/2019/2020 passed out candidates
Position:
Executive Trainee / Executive / Senior Executive
Date of Interview:
25-02-2021 (Thursday)
Interview Time:
9.00 AM to 11.00 AM
Only Male
Candidates
Venue Details:
MSN Laboratories Pvt. Ltd.,
R&D Center, Pashamylaram
Ph No : +91-8452304799/4899
040-30438786
Work Location : R&D Center, Pashamylaram.
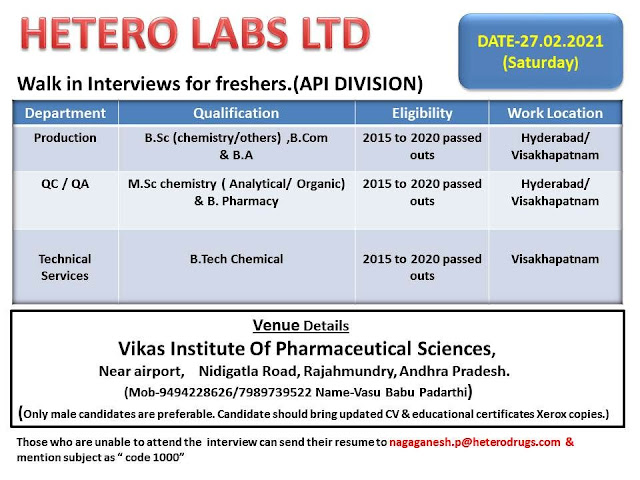
HETERO LABS LTD - Walk-In Interviews for FRESHERS in Production / QC / QA / R&D / Technical Services Department (TSD) on 27th Feb' 2021
Greetings from Hetero…!
Walk-in-interviews At Vikas Institute of Pharmaceutical Sciences,
Rajahmundry, East Godavari, A.P @ 27.02.2021
We are hiring Freshers for our API units which are located at Hyderabad &
Visakhapatnam areas.
Qualification- B.Sc ( Chemistry/ Others) , B.com & B.A
Department- Production
Exp- Freshers
Qualification- M.Sc Chemistry( Organic/ Analytical) & B.Pharmacy
Department- Quality control / Quality assurance / R&D
Exp- Freshers
Qualification- B.Tech Chemical
Department- TSD
Exp- Freshers
Eligibility- 1. 2015 to 2020 passed outs only
2. Male candidates are preferable
3. Candidate must speak telugu language
4. Willing to relocate Visakhapatnam & Hyderabad locations
Venue details- Vikas Institute of Pharmaceutical Sciences, Rajahmundry, East
Godavari, A.P
Date- 27.02.2021 (Saturday)
Regards,
Naga Ganesh Patnaik- HR
Hetero Corporate
70 Openings | Walk-In Drive for Regulatory Affairs on 6th Mar' 2021 @ MSN Laboratories Pvt. Ltd
Walk- In
Drive for Regulatory Affairs Dept. in API Division- R&D Center.
Regulatory Affairs API RA Dept. in API Division,
Openings: 70
Job Title: Executive / Senior Executive Experience: 1 to 8 years in API RA
field Education: B Pharma / M Pharma / M.Sc
Department: Regulatory Affairs
Job Profile:
1. Collecting and reviewing the documents received from various departments
R&D, AR&D, QA, QC and Production etc at each and every stage of
manufacturing of drug substances to minimize the errors at the time of submission
to regulatory agencies.
2. Hands on experience on the preparation of DMFs and their registration
processes in the major regulatory regions [US, Canada, Europe etc]..
3. Hands on experience on preparation of drug master files, Applicants Parts,
Tech Pack, life cycle management [updates and amendments] and drafting of
response to deficiencies and customer DMF review comments for all global
regions.
4. Should have hands on experience on eCTD regulatory submission tools.
5. Should have knowledge on the requirements to respond to the deficiencies.
6. Participation in the cross functional team meetings and providing regulatory
inputs to the Product Development Teams.
Job Title: Junior Manager / Assistant Manager Experience: 1 to 8 years in
API RA field Education: B Pharma / M Pharma / M.Sc
Department: Regulatory Affairs
Job Profile:
1. Good scientific conceptual background to the level to evaluate the processes
and procedures in the area of Quality, R&D, AR&D and Production.
2. Manageable communication in English [should be able to manage regulatory and
scientific discussions in the internal and external meetings and one to one
interactions within the organization and with customers]
3. Manageable knowledge on the regulatory and quality guidelines from various
regulatory agencies ICH, FDA, EMA, Health Canada, ANVISA, PMDA, MFDS, CFDA etc.
4. Thorough knowledge and hands on experience on the DMF registration and
marketing authorization application systems in the major regulatory regions
[US, Canada, Europe etc]..
5. Hands on experience on preparation of drug master files, life cycle
management and drafting of response to deficiencies and customer DMF review comments
for all global regions.
6. Should have knowledge on product developmental and the quality systems to ensure
the regulatory compliance.
7. Should have hands on experience on eCTD regulatory submission tools.
8. Conducting meetings for the deficiencies received from various regulatory
agencies and should have knowledge on the requirements to respond to the
deficiencies. Ensuring that the accurate and adequate responses are sent to the
authorities within the timelines defined by agencies.
9. Thorough review and ensuring that the regulatory submissions are adequate
and error free with minimum open issues and closing of open issues before filing
or before the receipt of deficiencies.
10. Participation in the cross functional team meetings and providing
regulatory inputs to the Product Development Teams.
11. Reviewing the documents received from various departments R&D,
AR&D, QA, QC and Production etc at each and every stage of manufacturing of
drug substances to minimize the errors at the time of submission to regulatory
agencies.
12. Review and assessment of change controls and providing guidance to the team
on proposed changes.
13. Ensuring that the regulatory databases are properly maintained and updated
on time to time for each regulatory activity.
Drug Regulatory Affairs API RA Dept.
Exp : 1 to 8
Years
Position: Junior Executive / Executive /Senior Executive / Junior Manager
Date of Interview:
06.03.2020 (Saturday)
Interview Time:
9.00 AM to 1.00 PM
Venue Details:
MSN Laboratories Pvt.Ltd.,
R&D Center, Pashamylaram
Ph No : +91-8452304799
040-30438786
Work Location : MSN LS II & R&D Center
HEREO - Openings for Regulatory Affairs - Bulk Drugs / API @ Hyderabad
REGULATORY AFFAIRS/EXPERIENCE-BULK DRUGS/API-HYDERABAD
Department: Regulatory
Affairs
Designation:
Executive/Sr. Executive
Industry: Pharma/API
Experience:
2 to 6 years
CTC: Negotiable
Job
Location: Sanath Nagar, Hyderabad
Roles and
Responsibilities
- Preparation of Drug Master Files (DMF) in CTD /
eCTD / Nees format for US, European Countries, Canada, Australia, Japan,
GCC, Turkey and Korea as per customer/ business requirement.
- Preparation of amendments/ Annual Reports to
USDMFs, CEP variations, EDMF Updates, Biannual updates to Canada DMFs and
Annual updates to Korea DMFs, Japan DMFs.
- Review and approval of change controls.
- Submission of notifications to the customers
pertaining to regulatory submissions as per the applicability.
- Responding to deficiency letters received from
various regulatory agencies like USFDA, EU Agencies, TGA, Health Canada
etc.
- Hands-on experience in Electronic
submissions-Lorenz.
- Maintaining query database and response
timelines.
- Tracking of variation submission timelines
(Amendments, Annual Reports) to various Health Authorities
- Responsible for organizing meetings to address
queries and variation submissions.
- Rendering customer support by preparing
technical packages, Applicant" s Part Drug Master Files and
responding to the technical queries received from customers.
- Preparation of pharmaceutical ingredients (API)
and its intermediates.
- Analytical interpretation, trouble shooting
& Plant performances.
Desired Candidates Please Share Your Updated Resume vivek.s@heterodrugs.com
HETERO - Urgent Openings for Freshers & Experienced in Production
Greetings from Hetero!!!
We have openings for Experienced Professionals at Jadcherla location.
Please find the details as mentioned below:
Department: Production (Injectable)
Designation: Operator
Area of Work: Compounding / Vial Washing / Componant / Filling
Required Experience: 2-5 Years
Qualification: B.Sc
Key Skills: Should have relevant pharma experience and hands on
experience in operating the machines.
Department: Production (Packing)
Qualification: B Pharmacy
Eligibility: 2018/19/ 20/ 21 Passed outs.
Designation: Junior Officer
Key Skills: Should have secured minimum 60% in academics and should
possess strong communication and analytical skills.
* Job Location: Jadcherla
* Interested candidates can drop their updated CV's at naveena.v@heterodrugs.com
Walk-In Drive for Freshers & Experienced in PQC (Plant / Quality Control) on 24th Feb' 2021 @ MSN Laboratories Pvt. Ltd
Walk- in
Drive for PQC - Dept .in API Division- R&D Center.
Eligibility Criteria:
Should have minimum of 0 to 3 years of experience handling in analytical
instrumentation HPLC, GC, GCMS, LCMS, in PQC/ Quality Control (QC) - Department
- API Division
Male
Candidate Only
Position:
Executive - Trainee / Executive
Department:
Plant / Quality Control (PQC)
Experience: 0 to 3 Years
Date of Interview:
24.02.2021 (Wednesday)
Interview Time:
9.00 AM to 11.00 AM
Venue
Details:
MSN Laboratories Pvt. Ltd.,
MSN R&D center, Pashamylaram, Isnapur, Patancheru, Sangareddy
040-30438786
Work Location: MSN R&D Center, Pashamylaram