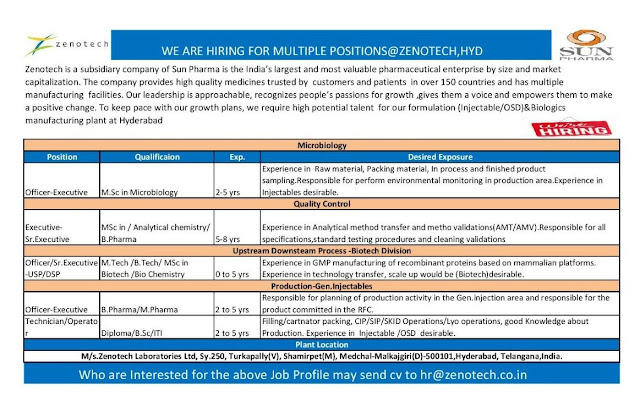
We are hiring for multiple positions, who are interested may send updated
cv to hr@zenotech.co.in
Saturday 12 December 2020
Zenotech Laboratories (Sun Pharma) - Multiple Openings for Microbiology / Quality Control / Production / Upstream & Downstream Process - Apply Now
Wednesday 9 December 2020
Syngene International Ltd - Virtual Recruitment Drive for M.Sc / M.Tech in Biotechnology, Biology, Biochemistry, Biomedical Sciences on 12th &19th December 2020
We are hiring! We are conducting virtual recruitment drive on 12th
&19th December 2020 for multiple openings in Discovery Biology for the
Genomics & Translational Sciences group & the Assay Biology group
respectively.
We are looking for talented scientists with experience in skillsets like
biochemical, biophysical (SPR) and cellular assays; Immunoassays, lymphocyte
analysis, coculture studies, functional characterization such as cell
proliferation, cytokine release, etc; Technology platforms (TR-FRET, AlphaLisa,
HTRF, Radiometric) and with automated platforms for screening in miniaturized
assay (96 and 384-well) formats for small or large molecules.
Details as below.
◆ Qualification & Experience: M.Sc/M.Tech in Biotechnology, Biology,
Biochemistry, Biomedical sciences with 3 to 10 years of relevant experience.
1. Req Id 15362- Associate Scientist- Immunology
Click 👉 https://bit.ly/3l7qe19 for
detailed Job Description
2. Req ID 19763- Associate Scientist- Assay Biology
Click 👉 https://bit.ly/39Wc8O0 for
detailed Job Description
3. Req ID 19765- Senior Research Associate- Assay Biology
Click 👉 https://bit.ly/2K7MzP8 for
detailed Job Description
◆ Job Location: Bengaluru
◆ Department: Discovery Biology
◆ For more details please write to Megha.paniker@syngeneintl.com
Tuesday 17 November 2020
Wednesday 4 November 2020
Accuprec Research Labs Pvt. Ltd - Telephonic / Virtual Interview for QA / QC / Microbiology / ADL / F&D / Biotechnology / Pharmacology & Toxicology / IT Departments - Apply Now
Departments: QA / QC / Microbiology / ADL / F&D / Biotechnology / Pharmacology & Toxicology / IT
Interested candidates send your updated Resume on career@accuprec.in along with mention the below details,
-Current CTC
-Onhand salary
-Expected Salary
-Notice Period
Sunday 1 November 2020
HETERO Biopharma - Urgent Openings for M.Sc / M.Tech / B.Tech in Biotech, Biochemistry, Microbiology Candidates - Production (Downstream Process)
HETERO Biopharma - Urgent Openings for M.Sc / M.Tech / B.Tech in Biotech, Biochemistry, Microbiology Candidates - Production (Downstream Process)
Tuesday 27 October 2020
Symbiotec Pharmalab (P) Ltd - Urgent Vacancy in Quality Control / Quality Assurance / Production / Biotech Departments - Apply Now
Vacancy in
Quality Control/ Quality Assurance/ API Production/ Biotech
Location:
Pithampur, Indore
Openings: 15
1) Quality
Control
Position-
Chemist/ Officer/ Sr. Officer
Experience-
2-6 Years
Qualification-BSc/M.Sc
Job
Description:
HPLC-
1.To perform and maintain calibration records of HPLC in QC lab.
2.To ensure proper and regular servicing of all the analytical
instruments.
3.To ensure updation of all HPLC log books.
4.To initiate and completion of analytical record for HPLC.
5.To carry out HPLC analysis of Raw-materials, finish products,
intermediates and working standards.
6.To perform and achieve departmental objectives.
7.To follow the guidelines of GLP and cGMP requirements.
8.To carry out instrumental analysis of stability samples
9.To maintain HPLC columns records.
10.To carry out chemical analysis of raw materials, finish products,
intermediates and working standards.
Wet Lab:
1.
To carry out the analysis of raw
materials, in- process, intermediates, packaging materials, finish products,
water & working standards.
2.
To updation of log books of raw
materials, in process, packaging materials and finish products.
3.
To updation of log books and preparation
of test solution and volumetric solution.
4.
To maintain all calibration records.
5.
To perform sampling of water, raw
materials, packaging materials, intermediates and finish products.
6.
Temperature monitoring of QC lab.
7.
To follow the guidelines of GLP and cGMP
requirements.
8.
To perform calibration of instruments
like pH meter, conductivity meter, Karl Fischer, Analytical Balance, LOD oven,
vacuum oven and UV analysis.
9.
To carry out chemical analysis of raw
materials, finish products, intermediates and working standards.
10.
Upkeep of samples under analysis and
retain samples.
11.
Upkeep of laboratory working area.
12.
Destruction of expired samples timely.
13.
To ensure adequate identification and
segregation of test samples to avoid mix up and cross contamination.
14.
Timely reporting of results of testing
to facilitate the achievement of production schedules.
15.
Discussion of out of specification
laboratory results with the manager, initiation of investigation of such
results and submission of additional results.
16.
To check report from the analyst against
standards and submission of inspected reports for review.
17.
To ensure that the records, which are
made of the results of inspection and testing of materials, intermediates and
finished products are formally assessed against specification.
2) API
Production:
Position-
Chemist/Officer/Sr. Officer
Experience-
2-8 Years
Qualification-BSc/M.Sc/BE
Job
Description:
1. Preparation of documents for production.
2. To co-ordinate with Production people and control manufacturing
activities.
3. To co-ordinate with other departments like maintenance, QC, warehouse
and QA etc. for smooth production.
4. To review production documents and check that are filled adequately
and signed.
5. To ensure that correct raw materials are issued for respective batch.
6. To check status on materials during all stages of operation.
7. To co-ordinate in reviewing the non-conformance of the product.
8. To ensure congenial and safe work environment.
9. To monitor production activities to meet the products requirements.
10. To perform and achieve department objectives.
3) Quality
Assurance-
QMS
Position-
Officer/ Sr. Officer/ Executive
Experience-
3-8
Qualification-BSc/M.Sc/Bpharm/
Mpharm
1. To issue correct version of BMR/BPR/PCOCR/MCVR and other documents.
2. To review filled Batch manufacturing record and analytical record.
3. To prepare the department SOP as and when required.
4. To maintain the records for FP/Intermediate batch release documents.
5. To prepare and review of the APQR.
6. To exercise document and data control whichever required.
7. To prepare and review of process validation and cleaning validation
protocols and reports.
8. To prepare and review of audit compliance.
9. To participate in self inspection / internal audit program.
10. To initiate, wherever essential and to handle change control,
deviations and incidents, CAPA, OOS, Market complaint.
11. To maintain the storage of master copies of all controlled documents.
12. To perform the work assigned by Manager-QA/Head-Quality.
IPQA-
1.
To issue correct version of
BMR/BPR/PCOCR/MCVR and other documents.
2.
To review filled Batch manufacturing
record and analytical record.
3.
Verification of work in progress of
manufacturing plant in accordance to approved BMR/BPR and SOPs.
4.
To verify the equipment/shop floor
cleaning and shall check the results of cleaning samples analysis during
changeover of product.
5.
To perform dispatch related activity.
6.
To verify packing activities of finished
product for dispatch as per OIS & SOP.
7.
To verify line clearance for next
product.
8.
To verify the calibration and
verification of weighing balance.
9.
To review filled Batch manufacturing
record and analytical record.
10. To prepare the department SOP as and
when required.
11. To exercise document and data control
whichever required.
12. To issue and retrieval controlled copies
of logbooks and monthly formats of all department.
13. To initiate, wherever essential and to
handle change control, deviations and incidents, CAPA.
14. To maintain the storage of master copies
of all controlled documents.
15. To verify dispensing activities of raw
material required for production of API.
4) Biotech
Inoculum-
Position-
Jr. Executive
Experience-
1-3 Years
Qualification- M.Sc. (Biochemistry OR Biotechnology OR Microbiology OR Life
Sciences)
1. To follow the SOPs of Inoculum lab and maintain the training record.
2. To monitor the lab equipments and record their status.
3. To perform verification and calibration of lab instruments.
4. To prepare the batch media and fill the batch manufacturing record.
5. To prepare culture media and sterility media.
6. To perform the sterility test.
7. To perform and record isolation and propagation and maintenance of
microbial culture
8. To perform the productivity test.
9. To follow the production planning.
10. To coordinate with QA department for issuance and submission of lab
records and for calibration and validation of lab equipments.
11. To coordinate with QC and Micro department for analysis, microbial
monitoring and GPT of media.
12. To coordinate with ware house department for issuance of batch media.
13. To coordinate with maintenance department for calibration and
validation of lab equipments.
14. To follow the GMP and GDP procedures in Inoculum lab.
15. To perform and achieve departmental objectives.
Fermentation-
Position- Jr.
Executive
Experience-
1-3 Years
Qualification:- M.Sc. (Biotechnology / Microbiology) OR B.Tech / M.Tech.
(Biotechnology)
1. Preparation of documents for production.
2. To co-ordinate with Production people and control manufacturing
activities.
3. To co-ordinate with other departments like maintenance, QC, warehouse
and QA etc. for smooth production.
4. To review production documents and check that are filled adequately
and signed.
5. To ensure that correct raw materials are issued for respective batch.
6. To check status on materials during all stages of operation.
7. To co-ordinate in reviewing the non-conformance of the product.
8. To ensure congenial and safe work environment.
9. To monitor production activities to meet the products requirements.
10. To perform and achieve department objectives.
Interested candidates please share CV on ruchi.kashyap@symbiotec.in